685f Mechanism of Thermal Decomposition of Furan
Both furan (C4H4O) and furfural (C4H3O-CHO) are important products in biomass pyrolysis. To understand the thermal cracking of furan, we have studied its thermal decomposition by entraining a few Torr in 1–2 atmospheres in a high temperature supersonic nozzle. Furan samples are heated to temperatures of 1000 – 1400 K for 30–50 Ásec. The decomposition products are identified by photoionization mass spectroscopy and infrared spectroscopy.
In the hyperthermal nozzle, the organic species such as furan are decomposed while entrained seeded in an inert gas (roughly 1 – 2 atm) through a resistively heated silicon carbide tube (1 mm ID, about 2 cm long, temperature up to 1800 K) into a high vacuum chamber. The injection of the gas pulses into the tube is supersonic, which is achieved by an increase in cross-sectional area upon going from the orifice in the pulsed valve (0.1 - 0.4 mm diameter) into the SiC tube. The near-sonic gas flow velocity through the tube allows a short contact time (roughly 30 Ásec), which is essential to avoid radical-radical reactions. The jet of organic fragments and the He carrier gas expands into a vacuum at 10-5 Torr. Subsequently, the beam of radicals impinges onto a 20 K CsI window forming a matrix for IR detection. Photoionization time-of-flight (TOF) mass spectroscopy is used to detect the species coming out of the nozzle and optimize the thermal dissociation conditions such as nozzle temperature, and length for maximum radical production. The TOF mass spectrometer uses the 9th harmonic of a YAG laser at 118.2 nm (or 10.487 eV) to photoionize the target species.
The thermal fragmentation of furan has been studied previously. In an early study, furan was passed through a heatable molecular flow reactor operating at low pressures of about 1 mTorr. Product analysis was continuously performed by an on-line quadrupole mass spectrometer with electron impact ionization. Furan was observed to decompose between 1050 and 1270 K by ring breakdown unimolecular reactions. Loss of carbon monoxide was the exclusive process. It was found that k░(1) is 1015.6 exp(-74 kcal mol-1/RT).
Instead of a flow tube, the thermal decomposition of furan was studied behind reflected shocks in a single shock tube, over the temperature range 1050-1460 K. Shocked samples were taken from the end block of the driven section and were analyzed on a Packard 800 series gas chromatograph using a flame ionization detector. Methylacetylene and carbon monoxide were the major reaction products of the furan decomposition reaction: CH3C╝CH + CO. The rate constant was measured to be 1015.25▒0.5 exp[-(77.5 ▒ 2.5) kcal mol-1/RT] s-l. In addition to (1), a second initiation reaction produces HCCH and CH2CO with a rate constant of 1014.7▒0.5 exp [-(77.5 ▒ 2.5) kcal mol-1/RT] s-l. Additional reaction products which appear in the pyrolysis were identified by their GC traces to be: CH2=C=CH2, C4H6, CH2=CH2, CH4, HCC-CCH, and C6H6. In a subsequent shock tube study, the vibrational relaxation, incubation times, and unimolecular dissociation of C4H4O was investigated over the extended temperature range 500-3000 K in 2-5% furan-krypton mixtures, 2% furan-neon mixtures, and in pure furan at pressures of 600 Torr. The experiments were performed in shock waves using laser-schlieren densitometry and time-of-flight mass spectrometry. Unimolecular dissociation is observed in mass spectrometry experiments between 1300 and 1700 K in a pressure range of 175-250 Torr as well as laser-schlieren experiments between 1700 and 3000 K for pressures between 100 and 600 Torr. The mass spectrometry experiments show that under the given conditions two molecular dissociation channels leading to (1) C3H4 + CO or to (2) HC╝CH + CH2CO are dominant. The branching ratio between these channels has been determined between 1300 and 1700 K. At low temperatures, the molecular channel leading to C3H4 and CO is preferred, but a channel switching was observed around 1700 K. When we decompose furan in a hyperthermal nozzle, no fragmentation is observed until nozzle temperatures of 1000 K are achieved. Residence times in the supersonic nozzle are 30–50 Ásec. The reaction products are identified by features in the PIMS at m/z 40 and 42. In separate experiments, infrared signatures of HCCCH3, CO, HCCH, and CH2CO have been detected when the reaction products are trapped in a cryogenic matrix. As the nozzle temperature is raised to 1400 K, the PIMS spectrum shows peaks at m/z 39 (the propargyl radical, HCCCH2) and m/z 41 (ketenyl radical, HCCO, or allyl radical, CH2CHCH2). If the concentration of furan in the fuel mixture is raised by a factor of 10, the PIMS spectrum reveals features at m/z 78 (benzene), m/z 94 (probably phenol, C6H5OH), and m/z 104 (perhaps styrene, C6H5CHCH2). In earlier work, at high nozzle pressures we have observed dimerization of propargyl radicals to generate benzene (HCCCH2 + HCCCH2 Ę C6H6). Consequently we believe that radical/radical processes are generating these higher molecular weight products. Ab initio electronic structure calculations have been carried out and these CBS-QB3 applications reveal that the a-C-H bond energy in furan is greater than benzene, DH298(a-C4H3O-H = 120 ▒ 2 kcal mol-1). Because the C-H bonds in furan are so strong, the initial step in the cracking of furan is likely rearrangement to a carbene which subsequently fragments to products. The mechanisms for furan cracking are certain to be complicated.  (1)
(1)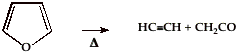 (2)
(2) (3)
(3)