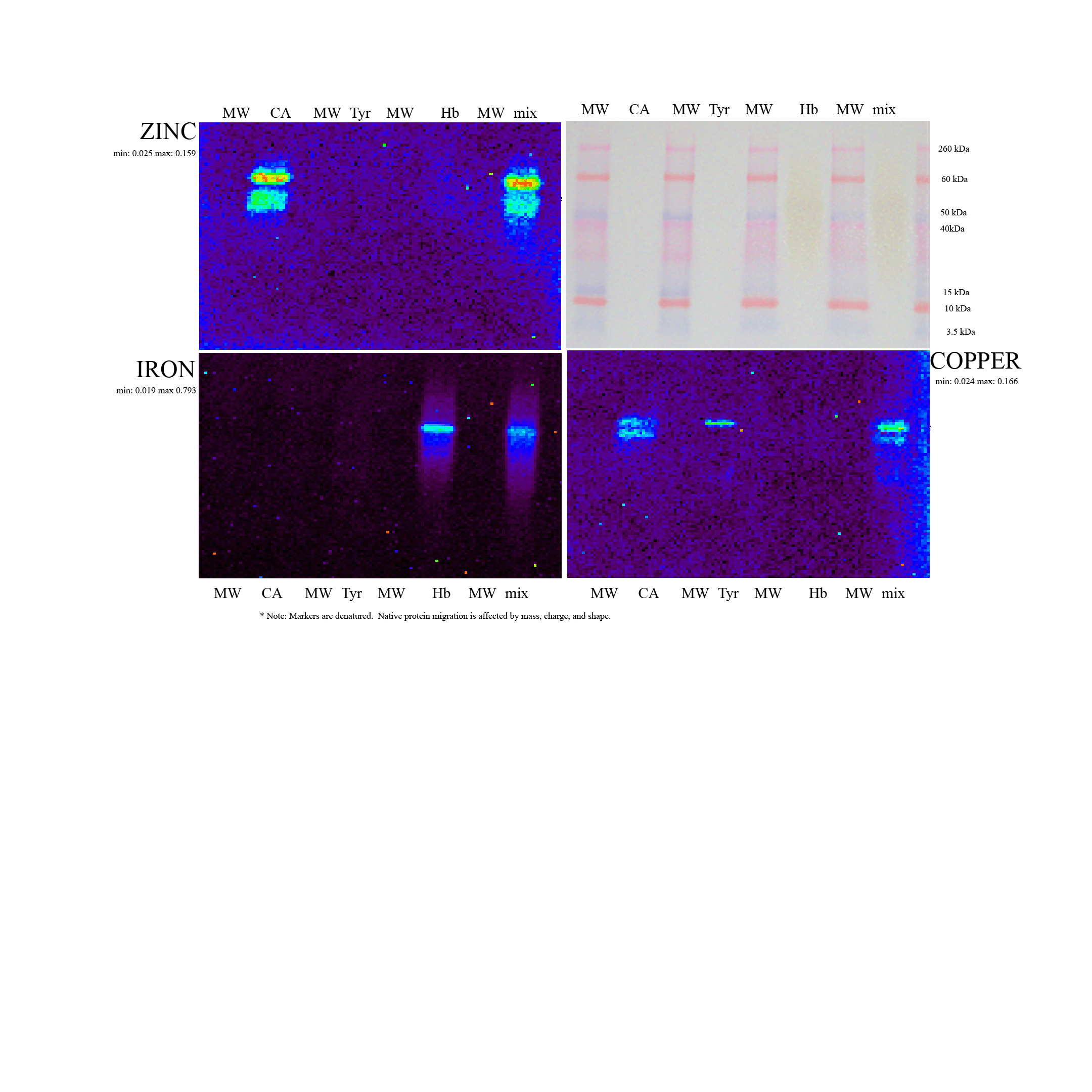
393b High-Throughput Metalloproteomics: X-Ray Fluorescence Imaging Paired with Electrophoresis
A critical need exists for technology that pairs with electrophoresis to identify and measure the metal ion component of metalloproteins in complex mixtures. These metals serve numerous roles as structural nuclei, catalytic cofactors, and in signaling. Their directed availability to proteins, a process itself tightly regulated, is a means of cellular control of protein activity. Yet, while gel electrophoresis allows us to readily capture information about proteins themselves, the metals they bind often elude comparable analysis. This work describes a simple, comprehensive approach for identifying and quantifying the metal ion component of metalloproteins in complex samples using native-PAGE and synchrotron x-ray fluorescence imaging. The approach is equally applicable to both 1D and 2D electrophoresis. Both the identification and quantitation of each metal bound to a protein spot is demonstrated, and the potential of this technique is illustrated in initial applications.