55h The Role of Phase Separated Nanodomains In the Solubility of Mixed-Monolayer-Protected Metal Nanoparticles
Self-assembled monolayer protected nanoparticles are promising candidates for applications, ranging from sensing to drug delivery, in which the molecular ligands' interactions with the surrounding environment play a crucial role. We recently showed (1-3) and present here that, when gold nanoparticles are coated with a binary mixture of immiscible ligands, ordered ribbon-like domains of alternating composition spontaneously form and that their width is comparable to the size of a single solvent molecule (fig. 1).
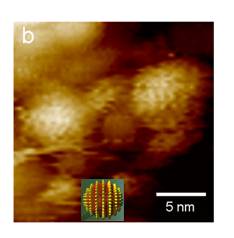 |
Fig.1: STM height images of 1-Octanethiol/Mercaptopropionic acid (2:1) nanoparticles. The striation presented on the latter are the signature of the formation of ribbon-like domains of alternating composition (3).
The ligand shell serves as the link between the nanoparticle and its environment, providing these materials with important properties such as stability and solubility. It is usually assumed that nanoparticles' solubility depends solely on the core size and on the molecular composition of the ligand shell. Here we show that this is not always the case. We find that the nanophase separation in the ligand shell morphology affects the solubility of these nanoparticles almost as much as the molecular composition. It is commonly believed that the solvent is responsible for the entropy of mixing (4), while the ligand shell molecules play the key role in determining the enthalpy of mixing due to the ligand-solvent, and the inter-particle ligand-ligand interactions. In the case of particles coated with mixture of ligands where each same ligand-ligand interaction is stronger that any hetero ligand-ligand interaction, one would expect the enthalpy of mixing for mixed ligand nanoparticles to be an average of the enthalpy of mixing of the component homoligand nanoparticles weighted over the composition of the ligand shell. Here we show that this is not the case when ribbon-like domains arise in the ligand shell and that the enthalpy further depends on the domain spacing (which can be varied through the stoichiometric ratio of the ligands used in the synthesis) (1). Indeed, the systematic studies on the solubility of nanoparticles presented as a function of composition in the ligand shell show non-monotonic behaviors indicative of physico-chemical effects that go beyond those expected by a simple thermodynamic treatment (1). We propose that it is likely that the ligand shell domains favor the wetting of the different solvents when their sizes and chemical groups match the solvent molecules size and chemical functionality thus contributing to the total NPs solubility.
1. A. M. Jackson, J. W. Myerson, F. Stellacci, Nature Materials 3, 330 (MAY, 2004).
2. A. M. Jackson, Y. Hu, P. J. Silva, F. Stellacci, Journal of the American Chemical Society 128, 11135 (AUG 30, 2006).
3. A. Centrone, Y. Hu, A. M. Jackson, G. Zerbi, F. Stellacci, Small 3, 814 (May, 2007).
4. G. Graziano, Journal of the Physical Society of Japan 69, 1566 (MAY, 2000).