766a High Unsaturated Alcohol Selectivity Over Silica and Alumina Pd-Sn Catalysts
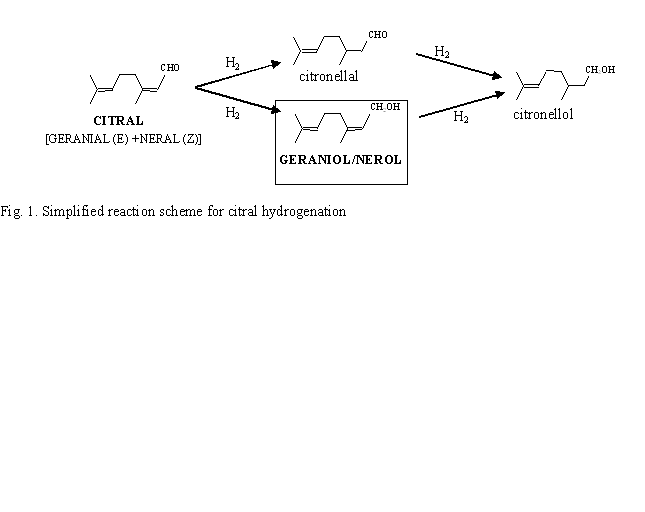
The selective hydrogenation of α,β-unsaturated aldehydes to their corresponding unsaturated alcohols is one of the most important research areas in fine chemistry. Among the group VIII metals, palladium is one of the worst metals for this reaction. In fact, Pd has been found highly selective (close to 100%) to the reduction of the conjugated C=C bond for different α,β–ethylenic aldehydes, such as citral, cinnamaldehyde and crotonaldehyde. In order to improve the selectivity toward the hydrogenation of the carbonyl bond, monometallic catalysts are usually modified either by support effects or by addition of a second metal. None of them, in the case of Pd, leads to an unsaturated alcohol selectivity higher than 45% (highest selectivity reported in the literature, obtained by using ZnO as support). In this work, the catalytic performances of two series of silica and alumina supported Pd-Sn catalysts have been studied in selective hydrogenation of citral (Fig. 1). Contrary to the literature dealing with Pd based catalysts, these bimetallic systems showed high selectivity (80%) towards the unsaturated alcohols (nerol and geraniol). To our knowledge, this is the first time that high unsaturated alcohol selectivities were obtained with Pd based catalysts. Different characterization methods (EDX coupled with TEM, TPR, cyclohexane dehydrogenation) as well as citral hydrogenation proved that tin is in great interaction with palladium. Hence, tin addition strongly modified the catalytic properties of palladium metallic phase. The current characterization of the catalysts by in-situ FT-IR and XPS will soon provide further insight into this structure-selectivity relationship.